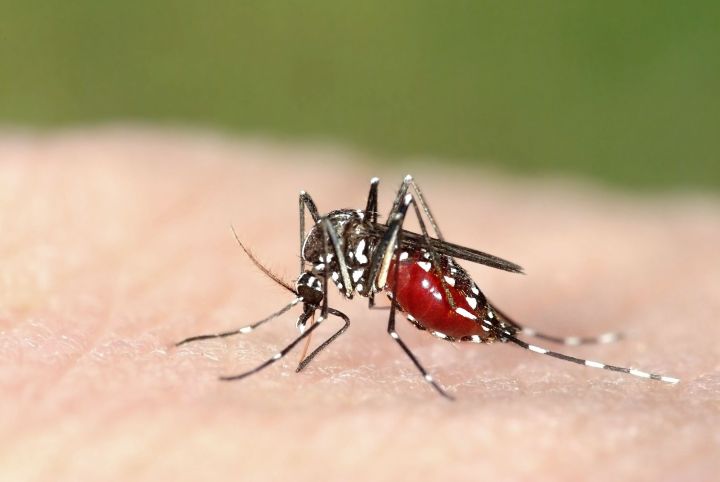
In a nutshell, gene driving is a method of manipulating DNA that ensures a gene is passed from a parent to its progeny at a rate as close as possible to 100 percent. To achieve this feat, scientists use the genome editing CRISPR–Cas9 system, which allows the researchers to target a specific area on the DNA for cutting and insertion of a mutated gene. This mutation is then transferred from one chromosome to another, ensuring all offspring inherit at least one copy of the modified gene. Because all offspring get a copy of the mutation, the modified gene then can be transmitted very quickly through a population of animals in the wild. As you can imagine, the technique is causing is the subject of much controversy among scientists. Not only does it override the natural process of evolution by quickly altering an population, these rapid changes can also produce unforeseen side effects on the ecosystem as a whole.
Earlier this year, developmental biologists Ethan Bier, Valentino Gantz, and their team from University of California, San Diego successfully engineered a gene drive in fruit flies. They then contacted Anthony James, a molecular biologist at the University of California, Irvine to see if their technique would apply to his research with malaria and mosquitos. Working with Bier and Gantz, James inserted two genes into a mosquito that would give the insect an innate resistance to the Plasmodium parasite. A follow-up study showed that the modified genes were passed to 99 percent of the mosquito’s offspring. The team confirmed the genes were being expressed in the progeny, but because their test was conducted in under laboratory conditions, they did not check to see if the genes conferred resistance as expected.
Well aware of the potential to change an entire population of insects, James experimented on a non-native mosquito, ensuring the mutation would not spread like wildfire in the rare chance a test mosquito escaped from the lab. Though happy with the outcome of his experiments, James confirmed he is in no rush to move his experiment from the laboratory to the field. “It’s not going to go anywhere until the social science advances to the point where we can handle it,” James says. “We’re not about to do anything foolish.”



