So why not reward them by teaching them a new, slightly more complex task — like carrying out an operation inside the human eye?
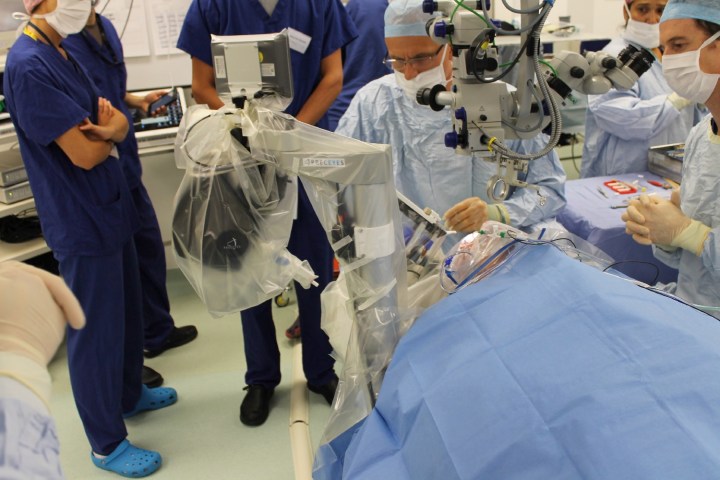
That’s exactly what a hospital in the United Kingdom did in a recent trial, in which robots competed against human surgeons to carry out a delicate surgery involving membrane-removal on the retina. A group of 12 patients was divided into two groups, with half undergoing the traditional human surgeon-led procedure and the other half undergoing the operation as carried out by robot.
“This is the first time robot-assisted surgery has been performed in the eye,” Marco Bellini, research coordinator of the Medical Sciences Division of Nuffield Department of Clinical Neurosciences, which carried out the project, told Digital Trends. “The robotic device is able to perform surgical procedures through the conventional surgical portholes used for retinal ‘keyhole’ surgery.”
The robotic surgical device itself was built by Preceyes, a Dutch engineering company. The advantage of the remotely-controlled robot is that it is able to more accurately perform extremely delicate operations, which even prove challenging for a trained surgeon to do by hand. The robot itself only has up/down, left/right, and toward the head/toward the feet directions, but within these parameters can operate with incredible accuracy; making movements as precise as 1 micron. In the eye, the robot operates through a single hole in the lens less than 1 millimeter in diameter, which it enters and exits several times during the procedure. The robot is controlled using a joystick and touchscreen by the human surgeon.
The combination of human and robot means carrying out the task with approximately 10x more precision than a human surgeon operating on their own. In the study, the patients in the robot group experienced significantly fewer hemorrhages, and less damage to the retina.
“Based on feedback from this pilot study, the Preceyes team is currently optimizing the prototype in order to increase its versatility and reliability,” Bellini said. “Once the device has been tested in more surgical procedures and is ready for market, an application will be made for a CE mark.” (Read: the mandatory marking found for certain products within the European Economic Area, similar to the FCC mark used on particular electronic devices in the United States.)


